The Hidden Battery in Your Mouth: Understanding Oral Galvanism
More and more people are becoming aware that the use of metal in dental restorations carries risks that extend far beyond the tooth itself.
Over time, these metals can corrode and leach into the mouth, where they are absorbed through the oral mucosa, swallowed, or even inhaled —
eventually entering the bloodstream and contributing to systemic toxicity.
Yet an equally concerning and often overlooked issue lies not just in the chemistry of these materials, but in their physics. When
dissimilar metals coexist in the mouth, they can interact through saliva to create a measurable electric current — a phenomenon known as
oral galvanism.
What Is Oral Galvanism?
The term galvanism refers to the generation of an electric current by chemical reaction between two different metals in an electrolyte
solution. In the oral cavity, that electrolyte is saliva — a conductive fluid rich in ions like sodium, chloride, and potassium.
When two different metals (for example, a mercury amalgam filling and a gold or stainless steel crown) are present in the same mouth, they
can form a galvanic cell. As these metals corrode and release ions into the saliva, an electric current is generated between them — much
like the electrodes of a small battery.
Shockingly, studies have measured oral galvanic voltages ranging from 100 to over 350 millivolts, with some reports of up to 3.5 volts in extreme cases (Mumford, J Dent Res, 1957; Certosimo & O'Connor, Gen Dent, 1996). These microcurrents can be 400–700 times stronger than the subtle electrical impulses used by the brain and nervous system for communication.
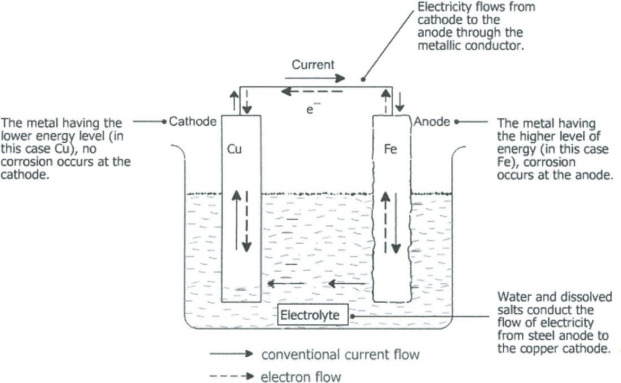
Source: https://www.sciencedirect.com/topics/engineering/galvanic-cell
Oral Galvanism Causes the Mouth to Become a Battery
When dissimilar metals such as mercury and gold are connected through saliva, electrons flow from the more "active" metal (the
anode) to the less active one (the cathode). This process, called galvanic corrosion, increases the rate of metal ion release and generates a
continuous electrical current.
This current can travel through the surrounding soft tissue, bone, and even nerve pathways. In the process, it can stimulate pain fibres in the pulp or mucosa — often experienced as a burning, metallic taste, tingling, or spontaneous discomfort without a clear cause. Oral galvanism can also cause electrical interference beyond the mouth. Here's how.
Oral Galvanism Can Cause Electrical Interference Beyond the Mouth
The human body is an intricate bioelectrical network — the brain, muscles, heart, and immune system all rely on finely tuned electrical
signalling to function. For instance, the heart's rhythm is governed by electrical impulses generated by the sinoatrial node, while neurons
in the brain communicate through rapid voltage changes across their membranes — known as action potentials — that transmit information
throughout the nervous system. Even immune cells use subtle bioelectrical cues to coordinate inflammatory responses and tissue repair
(Levin, Bioelectric Signalling: Reprogramming Cells and Regeneration, Cell, 2014).
Galvanic currents in the mouth, though small, can interfere with these signals. Research has proposed several mechanisms by which this occurs:
Neuroelectrical Interference
Oral microcurrents may disturb cranial nerve signalling, contributing to symptoms such as headaches, tinnitus, vertigo, or facial pain (Hyams & Ballon, CMAJ, 1933).
Electrochemical Sensitisation
Ion release can activate immune T-cells, triggering chronic inflammation or autoimmunity (Stejskal et al., Neuro Endocrinol Lett, 1999).
Systemic Allergic Reactions
Galvanic coupling between mercury amalgam and titanium implants has been shown to cause widespread dermatitis and immune dysfunction (Pigatto et al., Skin Allergy Meeting, 2010).
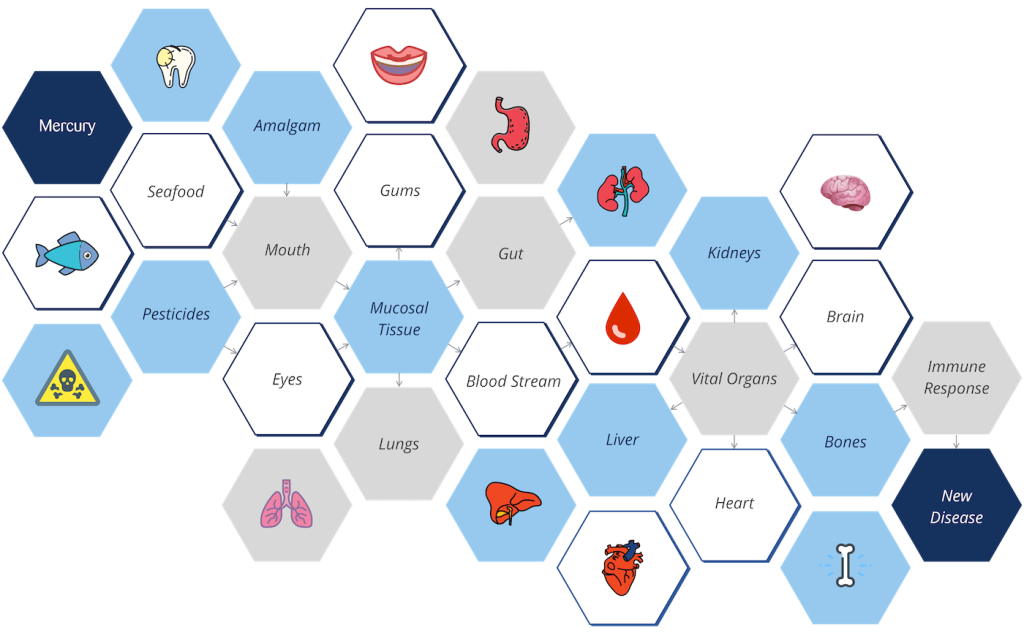
Mercury Doesn’t Just Stay in The Mouth
Once released from amalgam fillings, mercury is readily absorbed through mucosal tissues such as the gums, lungs, and gut. From there, it enters the bloodstream and travels to vital organs — including the kidneys, liver, brain, and heart.
Amplified by Modern Technology: EMF and Oral Galvanism
The effects of oral galvanism in modern society are amplified by our constant exposure to electromagnetic frequencies (EMF) from Wi-Fi, 5G
networks, Bluetooth devices, and countless digital systems woven into daily life.
Within this electromagnetic environment, the oral cavity can serve not only as a microbattery but also as a potential receiver and amplifier
of ambient electromagnetic radiation. In physics, any conductive loop with an electrical potential can act as an antenna. The human mouth,
when it contains multiple metals bathed in the ionic solution of saliva, satisfies exactly that condition.
Laboratory research on metal implants and dental alloys has shown that when exposed to radiofrequency (RF) fields, these conductive
materials can pick up and re-radiate electromagnetic energy — a principle long recognised in biomedical engineering (Ryu et al.,
Bioelectromagnetics, 2017). The result is localised current amplification, which may increase galvanic corrosion, heat nearby tissue, and
irritate sensory nerves.
In one experimental assessment, the magnitude of oral galvanic current was found to rise 400–700 times under fluctuating electromagnetic fields compared to a neutral baseline, suggesting that EMF exposure can significantly intensify intraoral electrical activity. As these microcurrents move through tissue rich in nerves and blood vessels, they can disrupt sensory balance, accelerate the release of metal ions, and compound the body's inflammatory burden.
As society grows ever more electrified, this interplay between dental metals and electromagnetic fields becomes increasingly relevant —
reminding us that the materials placed in the mouth do not exist in isolation, but interact dynamically with the invisible electrical
environment that now surrounds us all.
Clinical Manifestations and Diagnosis
The symptoms of oral galvanism vary widely, reflecting both local irritation and systemic electrical disturbance. Some effects are confined to the mouth, while others involve the broader nervous and immune systems. Because the oral cavity is richly innervated and connected to the cranial nerves, even small galvanic currents can influence distant physiological processes.
Common symptoms associated with oral galvanism include:
- Burning or metallic taste in the mouth
- Oral lichen planus or other mucosal lesions
- Chronic headaches or facial neuralgia
- Tinnitus, dizziness, or visual disturbances
- Chronic fatigue or unexplained systemic inflammation
Beyond these general manifestations, many people experience symptoms that point directly to nervous system interference. The low-level electrical currents generated by dissimilar metals can alter neural activity, muscle tone, and sensory perception in subtle yet pervasive ways.
Neurological and Nervous System Effects
Trigeminal Neuralgia and Facial Paresthesia
The trigeminal nerve, which supplies sensation to much of the face and oral cavity, is particularly susceptible to galvanic microcurrents.
Patients may describe sharp, electric-like pain, tingling, or numbness in the jaw and cheeks—symptoms that often subside after removal of
the offending metals (Mumford, J Dent Res, 1957).
Neuromuscular Dysfunction
Chronic electrical stimulation can affect neuromuscular junctions, causing involuntary facial muscle twitching, jaw tightness, or bruxism.
These symptoms mirror those seen in chronic low-level electrical exposure.
Autonomic Dysregulation
Persistent current flow near cranial nerve centres may disturb the autonomic nervous system, leading to palpitations, dizziness,
temperature sensitivity, or episodes of anxiety and panic.
Cognitive and Neuropsychiatric Symptoms
Metal ions such as mercury, nickel, and palladium released by galvanic corrosion are neurotoxic and can cross the blood–brain barrier.
Patients have reported "brain fog," memory lapses, difficulty concentrating, and mood disturbances, likely linked to disruption
of the hypothalamic–pituitary–adrenal (HPA) axis (Stejskal et al., Neuro Endocrinol Lett, 1999).
Sensory Disturbances and Tinnitus
Electrical interference transmitted through the trigeminal and auditory nerve pathways can result in tinnitus, vertigo, or pressure
sensations in the head and ears (Certosimo & O'Connor, Gen Dent, 1996).

How Mercury Causes Depression and Anxiety
Mercury promotes the overproduction of free radicals and depletes the body’s antioxidant defences — resulting in oxidative stress. The brain, with its high oxygen consumption and lipid-rich composition, is especially vulnerable to this process, which can culminate in depression and anxiety.
The Challenge With Diagnosis
Because the above symptoms often mimic other neurological or systemic disorders, oral galvanism frequently goes undetected — especially in the context of modern medicine and dentistry. Dentists trained in biological or holistic methods may use a multimeter to measure potential differences between restorations in millivolts (mV) or microamperes (µA). This is commonly the only path for detection.
Removal and Recovery
When significant galvanic activity is identified, sequential removal of incompatible materials often leads to measurable improvement in comfort, energy, and neurological stability.
In an extensive clinical study of 513 patients, Stejskal et al. (2006) found that replacing incompatible metals significantly reduced
metal-reactive lymphocytes and improved systemic health. Another study (Neuro Endocrinol Lett, 2006) confirmed that metal-specific immune
activity decreased after dental metal replacement, supporting a causal link between galvanic corrosion and immune activation.
Similarly, in our clinic, patients often report improvements in fatigue, mood, cognition, and pain after eliminating mixed-metal
restorations — not only because of the systemic toxic burden, but because an ongoing electrochemical disturbance is finally resolved.
The Safe Removal of Amalgam Fillings
One of the keys to the safe removal of amalgam fillings is to assess the electrical charge of each quadrant of the mouth before removal.
Amalgams should then be removed in order from the most negatively charged to the most positively charged quadrant.
What to do if You Have Multiple Metals in Your Mouth
If you have multiple metal restorations — such as mercury amalgam fillings alongside gold, titanium, or stainless-steel crowns — you may be at risk of the effects of oral galvanism.
The first and most crucial step is to work with a qualified biological or holistic dentist who has experience in assessing galvanic currents
and material compatibility. Through simple testing, they can determine whether electrical activity exists between your restorations and
evaluate your overall systemic sensitivity to metals.
If removal is warranted, it must be done strategically and safely. At our clinic, we only remove mercury amalgam and other reactive
materials as part of our Total
Dental Revision
program -- a comprehensive health protocol that includes:
- Detoxification support, to bind and eliminate circulating heavy metals
- Blood chemistry and mineral balance assessment, to ensure the body's detox pathways are functioning optimally
- Sequential removal, guided by measured galvanic currents to minimise exposure and systemic stress
Without such precautions, the removal process itself can mobilise stored toxins and temporarily worsen symptoms — a common oversight when
restorations are replaced without biochemical preparation.

